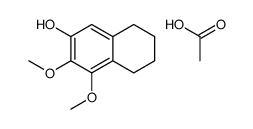
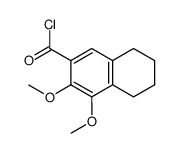
|
~84% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~85% |
|
~67% |
|
~% |
|
~% |
|
~76% |
|
~% |
|
~% |
|
~75% |
|
~69% |
|
~% |
|
~% |
|
~% |
|
~% |



![1,2,3,4-Tetrahydro-6-methoxy-2-[(4-methoxyphenyl)methyl]isoquinolin-7-ol Structure](https://image.chemsrc.com/caspic/236/5056-80-4.png)
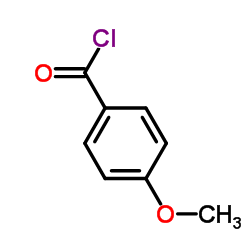
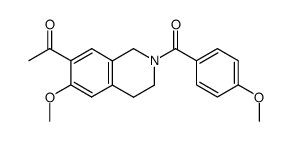

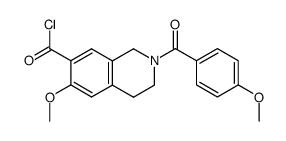
![methyl 6-(4-methoxybenzoyl)-2-oxo-5,6,7,8-tetrahydro-2H-pyrano[3,2-c]pyridine-3-carboxylate Structure](https://image.chemsrc.com/caspic/411/91586-27-5.png)