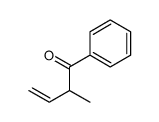
|
~83% |
|
~99% |
|
~8% |
|
~65% |
|
~71% |
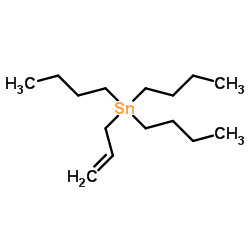
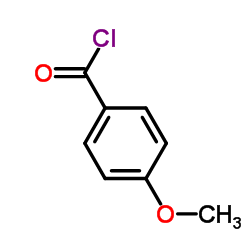
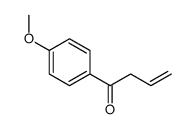
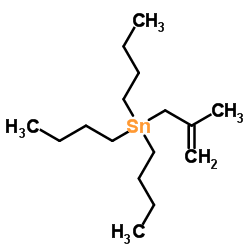


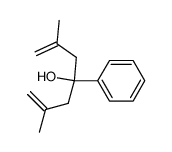
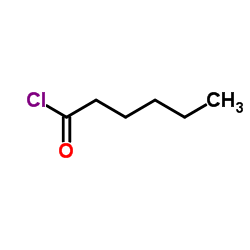
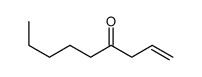
![but-2-enyl-[(tri-n-butyl)]-stannane Structure](https://image.chemsrc.com/caspic/311/35998-93-7.png)