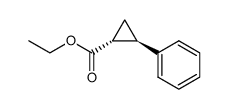
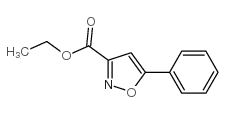
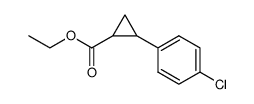
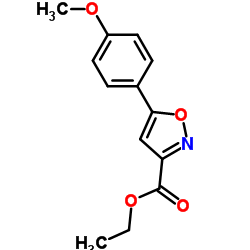
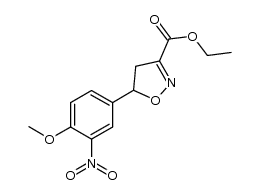
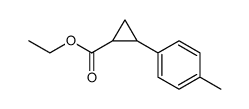
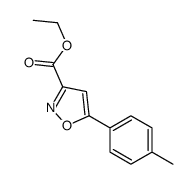
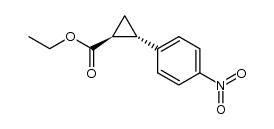
|
~88% |
|
~78% |
|
~83% |
|
~21% |
|
~% |
|
~95% |
|
~84% |
|
~% |