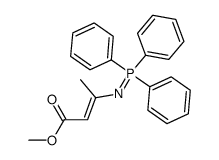
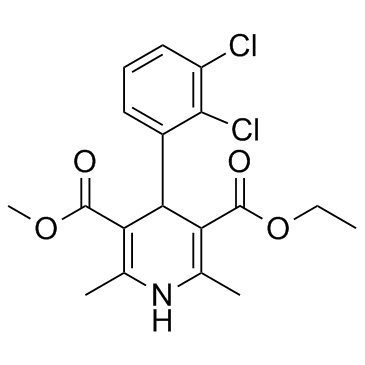
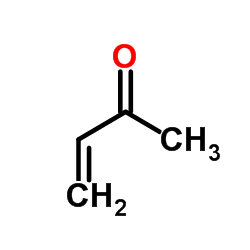
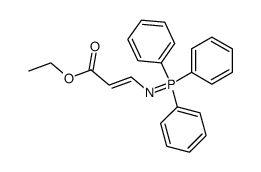
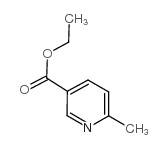
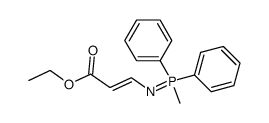
|
~84% |
|
~39% |
|
~15% |
|
~8% |
![2-[(2,3-dichlorophenyl)methylene]-3-oxobutanoic acid ethyl ester Structure](https://image.chemsrc.com/caspic/104/68064-64-2.png)