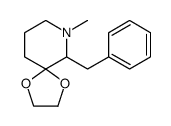
|
~% |
|
~% |
|
~73% |
|
~74% |
|
~% |
|
~% |
|
~% |
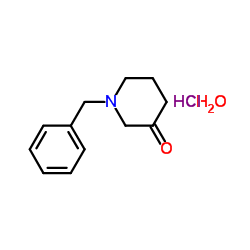
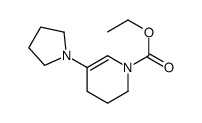
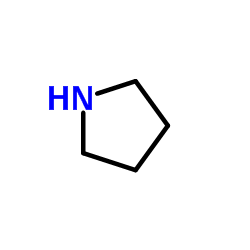
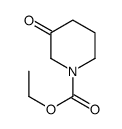
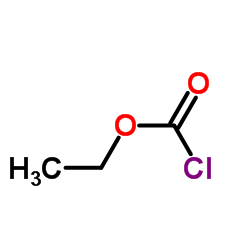
![ethyl 6-benzyl-1,4-dioxa-7-azaspiro[4.5]decane-7-carboxylate Structure](https://image.chemsrc.com/caspic/421/102520-49-0.png)