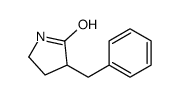
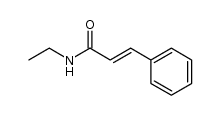
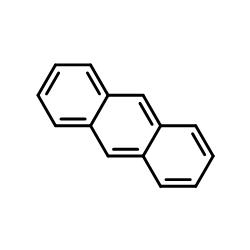
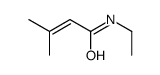
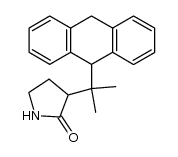
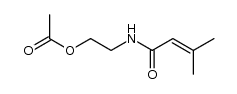
|
~86% |
|
~54% |
|
~44% |
|
~45% |
|
~%
Detail
|