|
~79% |
|
~% |
|
~% |
|
~80% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~90% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~61% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~76% |
|
~% |
|
~% |
|
~33% |
|
~% |
|
~95% |
|
~97% |
|
~% |
|
~88% |
|
~% |
|
~% |
|
~42% |
|
~% |
|
~% |
|
~78% |
|
~% |
|
~% |
|
~95% |
|
~% |
|
~% |
|
~91% |
|
~% |
|
~% |
|
~% |
|
~93% |
|
~% |
|
~% |
|
~81% |
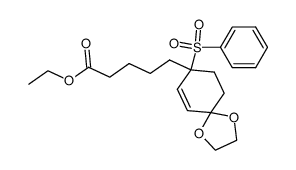
![ethyl 5-[1-(benzenesulfonyl)-4-oxocyclohex-2-en-1-yl]pentanoate Structure](https://image.chemsrc.com/caspic/052/81842-38-8.png)
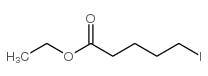
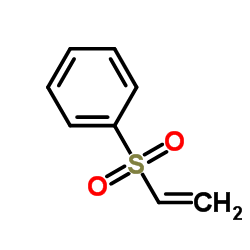
![4-oxo-3-methyl-1-(phenylsulfonyl)-cis-bicyclo[4.3.0]non-2-ene Structure](https://image.chemsrc.com/caspic/025/84065-59-8.png)
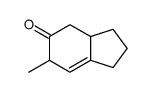
![(E)-3-methyl-4-methoxy-2-[(trimethylsilyl)oxy]-1,3-butadiene Structure](https://image.chemsrc.com/caspic/307/72476-03-0.png)
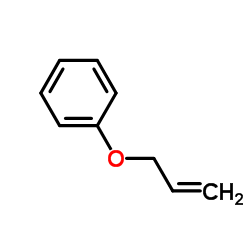
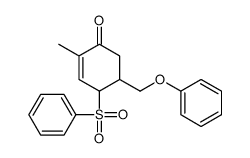
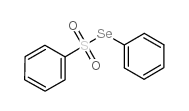
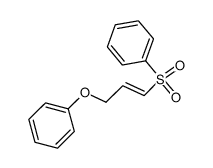
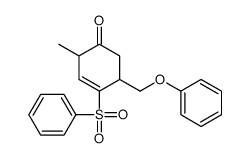
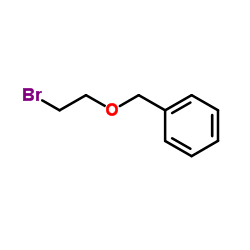
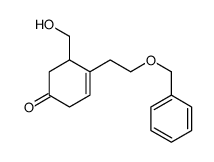
![tert-butyldimethyl((8-(phenylsulfonyl)-1,4-dioxaspiro[4.5]dec-9-en-7-yl)methoxy)silane Structure](https://image.chemsrc.com/caspic/479/87640-87-7.png)
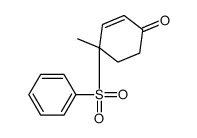
![8-(benzenesulfonyl)-8-methyl-1,4-dioxaspiro[4.5]dec-6-ene Structure](https://image.chemsrc.com/caspic/412/81841-91-0.png)
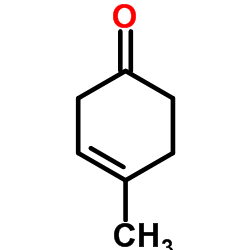
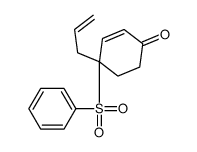
![8-(benzenesulfonyl)-8-prop-2-enyl-1,4-dioxaspiro[4.5]dec-6-ene Structure](https://image.chemsrc.com/caspic/374/81841-92-1.png)
![6,6-(ethylenedioxy)-3-[3,3-(ethylenedioxy)butyl]-3-(phenylsulfonyl)cyclohexene Structure](https://image.chemsrc.com/caspic/111/81842-41-3.png)
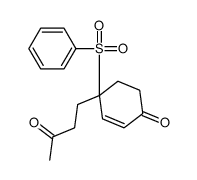


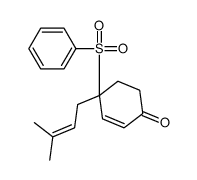
![8-(benzenesulfonyl)-8-(3-methylbut-2-enyl)-1,4-dioxaspiro[4.5]dec-6-ene Structure](https://image.chemsrc.com/caspic/336/81841-93-2.png)
![8-(benzenesulfonyl)-8-benzyl-1,4-dioxaspiro[4.5]dec-6-ene Structure](https://image.chemsrc.com/caspic/298/81841-94-3.png)
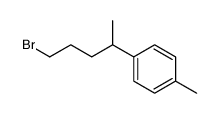
![4-[4-(4-methylphenyl)pentyl]cyclohex-2-en-1-one Structure](https://image.chemsrc.com/caspic/373/81842-25-3.png)
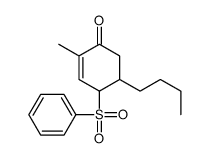
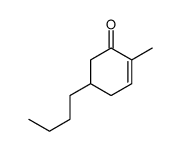
![[(trans-hex-1-ene)-1-sulfonyl]benzene Structure](https://image.chemsrc.com/caspic/490/68969-25-5.png)
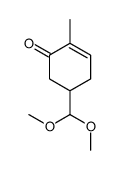
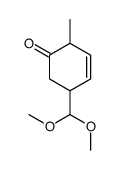
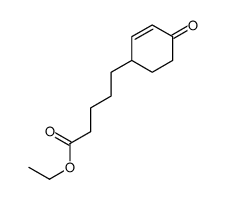
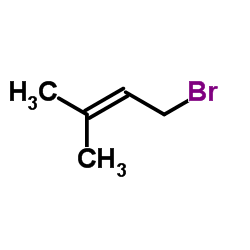

![4-(benzenesulfonyl)-4-[4-(4-methylphenyl)pentyl]cyclohex-2-en-1-one Structure](https://image.chemsrc.com/caspic/204/81842-34-4.png)
![6,6-(ethylenedioxy)-3-[3-(phenylthio)propyl]-3-(phenylsulfonyl)cyclohexene Structure](https://image.chemsrc.com/caspic/222/81841-96-5.png)
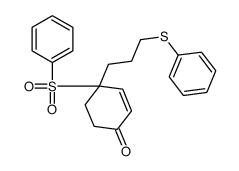
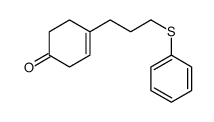
![4-[4-(4-methylphenyl)pentyl]cyclohex-3-en-1-one Structure](https://image.chemsrc.com/caspic/428/81842-19-5.png)
![8-(3-phenylsulfanylpropyl)-1,4-dioxaspiro[4.5]dec-7-ene Structure](https://image.chemsrc.com/caspic/335/81842-04-8.png)
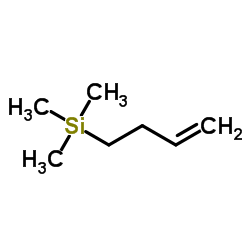
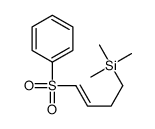
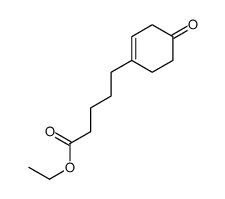
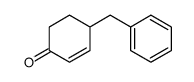
![1-(phenylsulfonyl)-3-methyl-4-oxo-8-oxa-cis-bicyclo[4.3.0]non-2-ene Structure](https://image.chemsrc.com/caspic/063/84065-58-7.png)