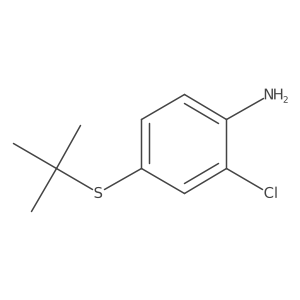
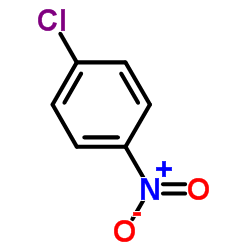
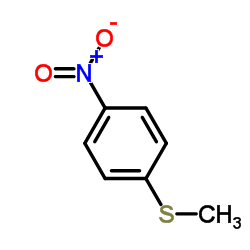
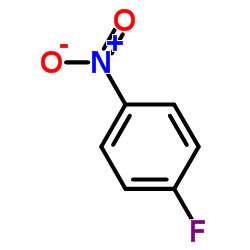
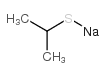
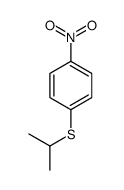
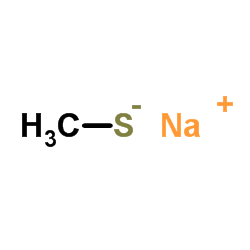|
~0%
Detail
|
|
~99% |
|
~99% |
|
~% |
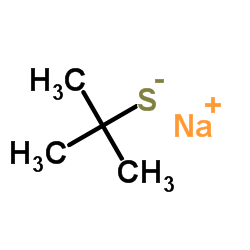
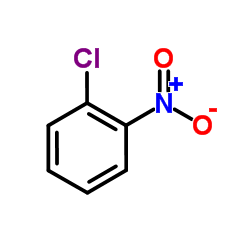
![2-[(2-Methyl-2-propanyl)sulfanyl]aniline Structure](https://image.chemsrc.com/caspic/465/51942-41-7.png)