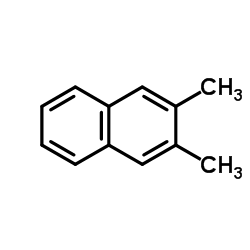
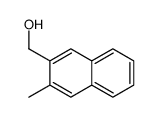
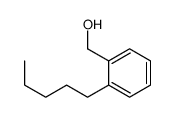
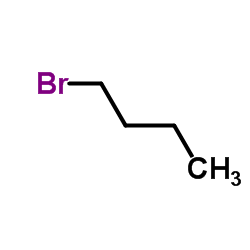
|
~% |
|
~69% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~56% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
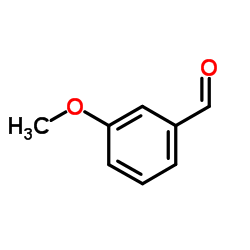
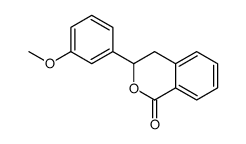
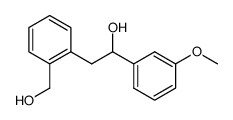
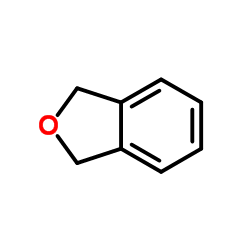


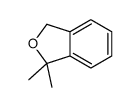
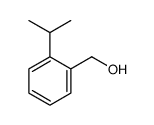

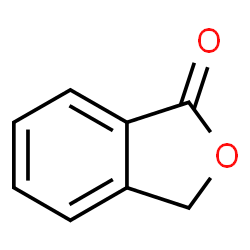
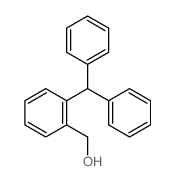

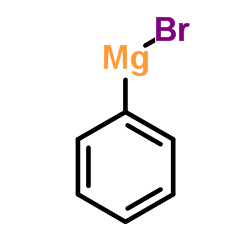
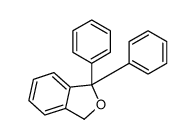
![2-[2-(hydroxymethyl)phenyl]-1-phenyl-ethanol Structure](https://image.chemsrc.com/caspic/085/38453-85-9.png)
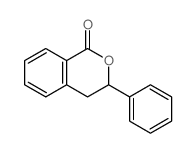

![1,3-dihydronaphtho[2,3-c]furan Structure](https://image.chemsrc.com/caspic/345/7193-16-0.png)