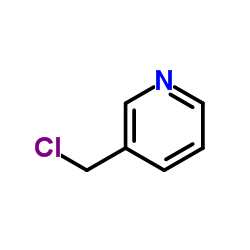
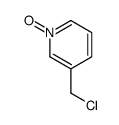
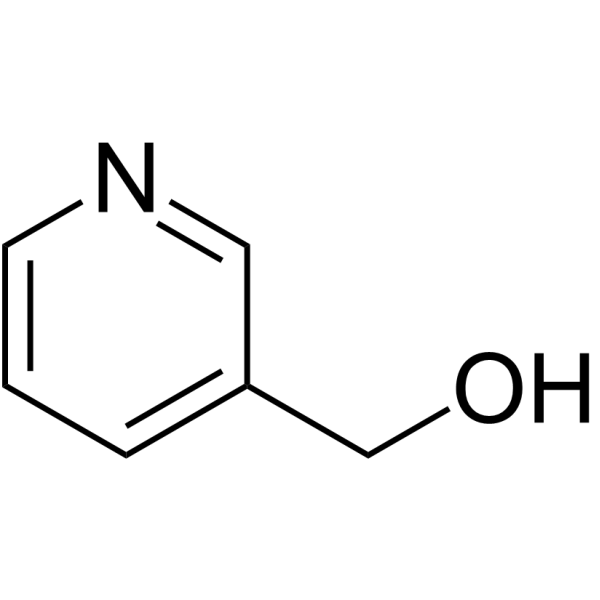
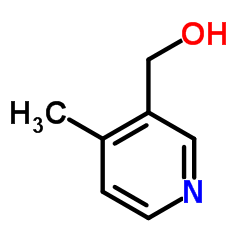
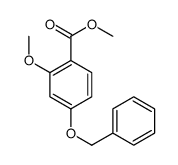
|
~% |
|
~% |
|
~99% |
|
~% |
|
~% |
|
~73% |
|
~% |
|
~% |
|
~% |
|
~% |
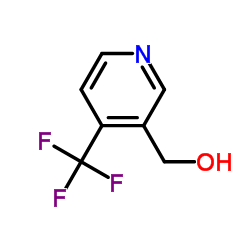
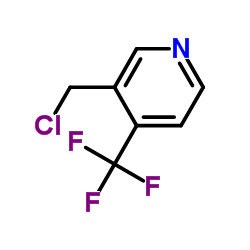
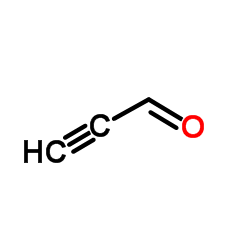

![6,7-Dihydro-5H-cyclopenta[b]pyridin-5-one Structure](https://image.chemsrc.com/caspic/057/28566-14-5.png)
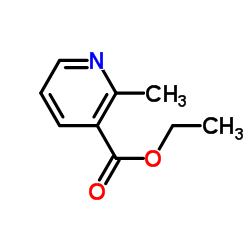
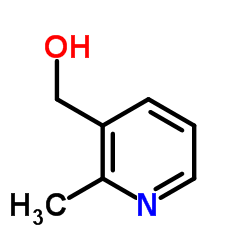
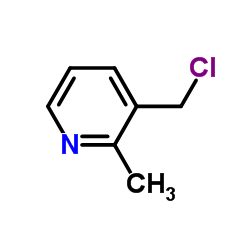
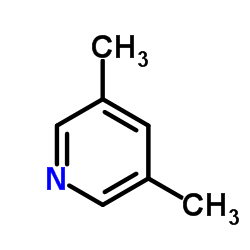
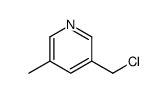
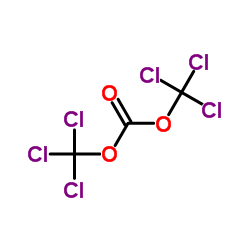
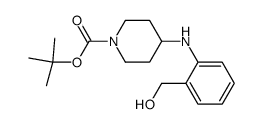
![TERT-BUTYL4-(2-OXO-2,4-DIHYDRO-1H-BENZO[D][1,3]OXAZIN-1-YL)PIPERIDINE-1-CARBOXYLATE Structure](https://image.chemsrc.com/caspic/198/162045-30-9.png)