|
~97% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~91% |

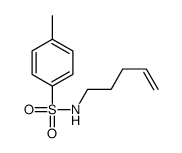
![6-bromobenzo[1,3]dioxole-5-carboxylic acid Structure](https://image.chemsrc.com/caspic/024/60546-62-5.png)
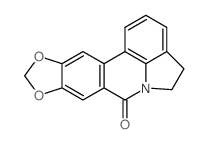

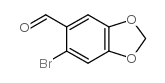
![methyl 5-(6-bromobenzo[d][1,3]dioxole-5-carboxamido)furan-2-carboxylate Structure](https://image.chemsrc.com/caspic/386/208335-55-1.png)