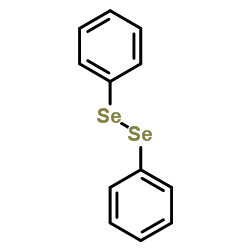
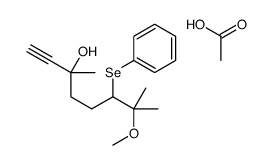
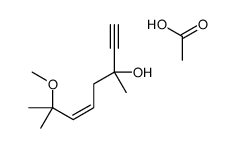
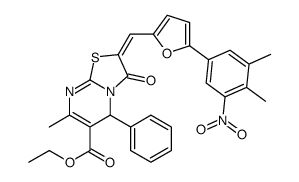
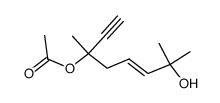
|
~91% |
|
~% |
|
~64%
Detail
|
|
~% |
|
~% |