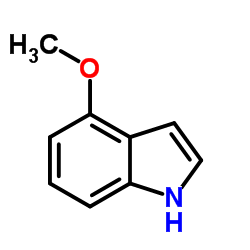
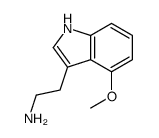
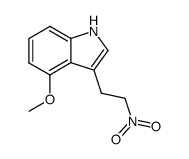
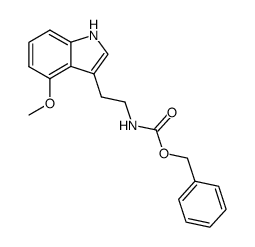
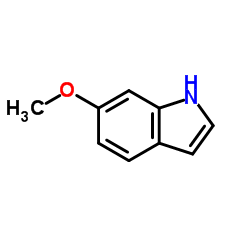
|
~85% |
|
~49% |
|
~95% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~26% |
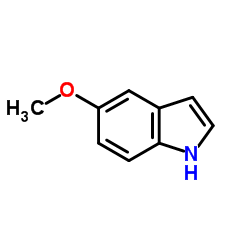
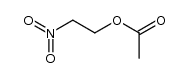

![Pyrrolidine, 1-[2-(3-methoxy-2-nitrophenyl)ethenyl] Structure](https://image.chemsrc.com/caspic/409/96096-68-3.png)
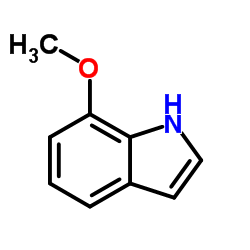
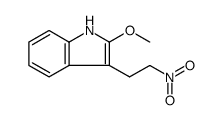
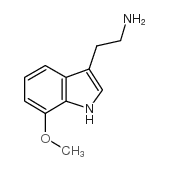

![N-[2-(5-methoxy-1H-indol-3-yl)ethyl]benzyloxycarboxamide Structure](https://image.chemsrc.com/caspic/023/96096-65-0.png)
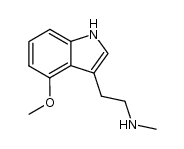

![N-[2-(4-Methoxy-1H-indol-3-yl)ethyl]-N-methyl-2-propanamine Structure](https://image.chemsrc.com/caspic/316/96096-53-6.png)