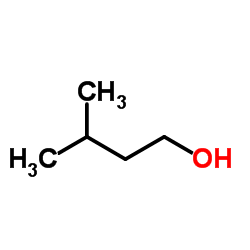
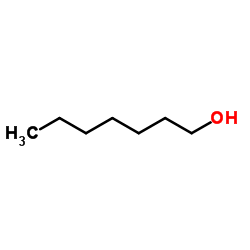
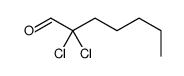
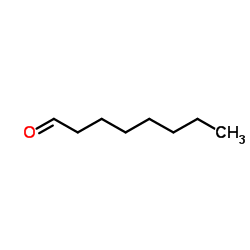
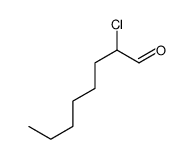
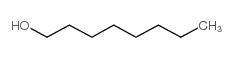
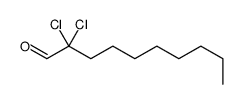
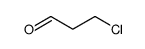
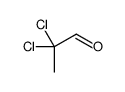
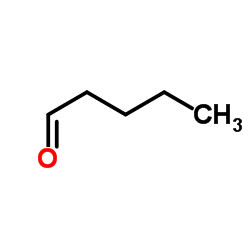
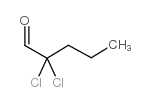
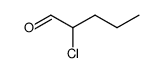
|
~83% |
|
~90% |
|
~1% |
|
~91% |
|
~95% |
|
~91% |
|
~0% |
|
~% |