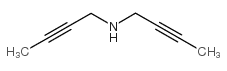
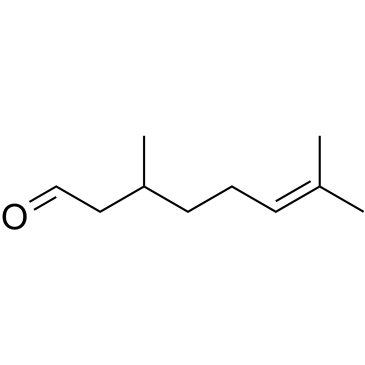
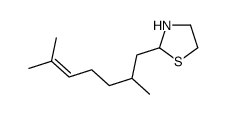
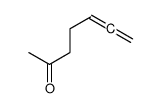
|
~20% |
|
~75% |
|
~60% |
|
~82% |
|
~50% |
|
~58% |
|
~70% |
|
~78% |
|
~52% |
|
~71% |
|
~25% |
|
~43% |
|
~62% |
|
~45% |
|
~74% |
|
~82% |
|
~59% |
|
~50% |
|
~65% |
|
~76% |
|
~76% |
|
~73% |
|
~69% |
|
~73% |
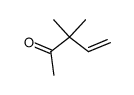
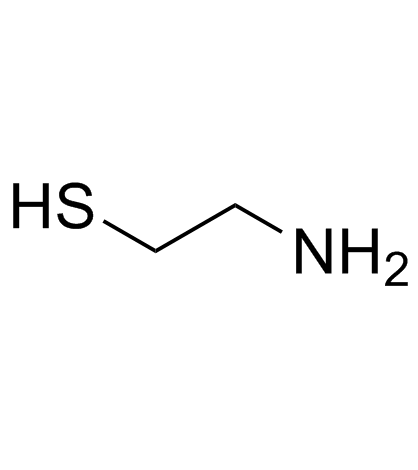
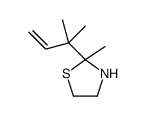
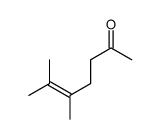
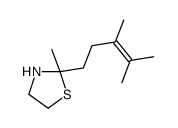
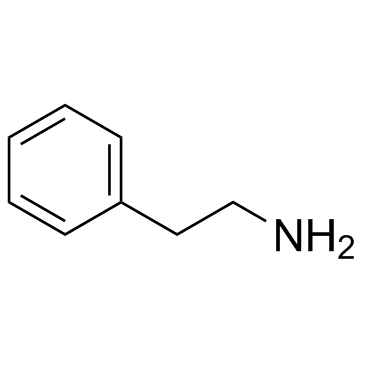
![Ethanethiol,2-[(2-phenylethyl)amino] Structure](https://image.chemsrc.com/caspic/125/6197-28-0.png)
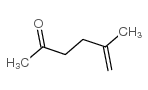
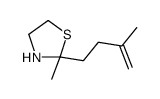
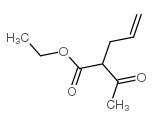
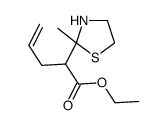
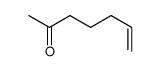

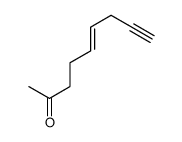
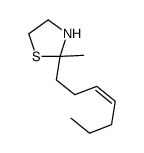
![2-[(E)-hept-3-en-6-ynyl]-2-methyl-1,3-thiazolidine Structure](https://image.chemsrc.com/caspic/433/75606-69-8.png)

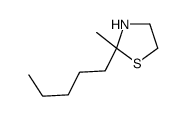
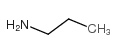

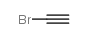
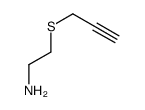
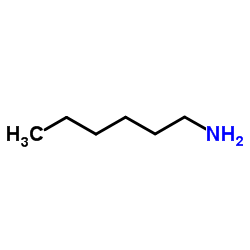

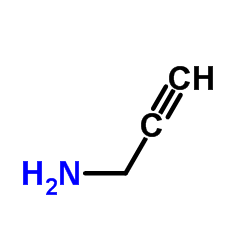
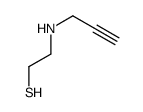
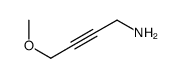
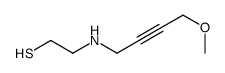
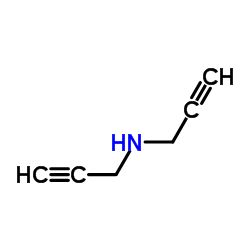
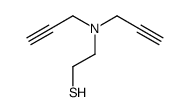
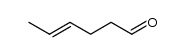
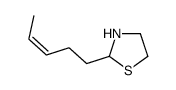


![2-[(3-Phenylpropyl)amino]ethanethiol Structure](https://image.chemsrc.com/caspic/097/7081-80-3.png)
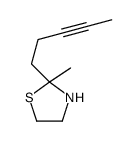
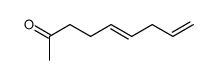
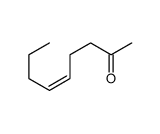
![2-[(3E)-hepta-3,6-dienyl]-2-methyl-thiazolidine Structure](https://image.chemsrc.com/caspic/471/75606-68-7.png)