|
~87% |
|
~% |
|
~85% |
|
~% |
|
~% |
|
~80% |
|
~% |
|
~% |
|
~95% |
|
~% |
|
~% |
|
~90% |
|
~91% |
|
~% |
|
~% |
|
~84% |
|
~% |
|
~% |
|
~% |
|
~92% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~93% |
|
~94% |
|
~96% |
|
~90% |
|
~% |
|
~78% |

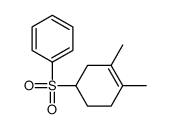
![[1-(benzenesulfonyl)-3,4-dimethylcyclohex-3-en-1-yl]methylbenzene Structure](https://image.chemsrc.com/caspic/239/73301-21-0.png)

![[1-(benzenesulfonyl)-4-methylcyclohex-3-en-1-yl]-trimethylsilane Structure](https://image.chemsrc.com/caspic/049/73301-26-5.png)
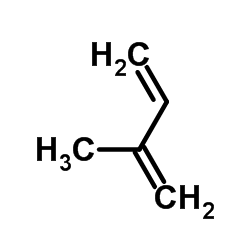
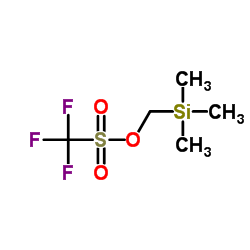
![[1-(benzenesulfonyl)-4-methylcyclohex-3-en-1-yl]methyl-trimethylsilane Structure](https://image.chemsrc.com/caspic/011/73301-27-6.png)
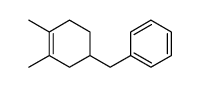
![exo-1-methoxy-6-(phenylsulfonyl)bicyclo[2.2.2]oct-2-ene Structure](https://image.chemsrc.com/caspic/218/73301-17-4.png)
![4-methoxybicyclo[2.2.2]oct-2-ene Structure](https://image.chemsrc.com/caspic/417/25489-02-5.png)
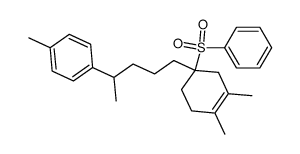
![1-[5-(3,4-dimethylcyclohex-3-en-1-yl)pentan-2-yl]-4-methylbenzene Structure](https://image.chemsrc.com/caspic/032/73301-31-2.png)
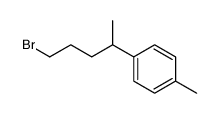
![2-[(4-methylcyclohex-3-en-1-yl)methyl]oxolane Structure](https://image.chemsrc.com/caspic/380/73301-35-6.png)
![trimethyl-[(4-methylcyclohex-3-en-1-yl)methyl]silane Structure](https://image.chemsrc.com/caspic/342/73301-36-7.png)

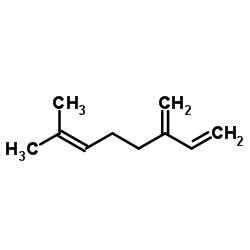
![[4-(4-methylpent-3-enyl)cyclohex-3-en-1-yl]sulfonylbenzene Structure](https://image.chemsrc.com/caspic/256/73301-16-3.png)
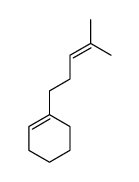
![5-(phenylsulfonyl)bicyclo[2.2.1]hept-2-ene Structure](https://image.chemsrc.com/caspic/454/794592-27-1.png)
