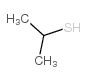
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
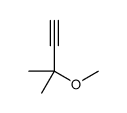

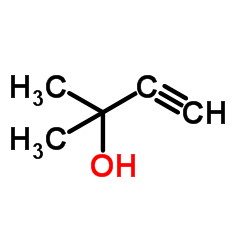
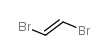
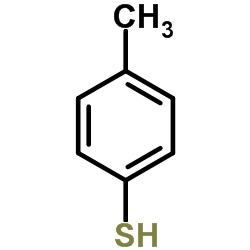
![1-methyl-4-[2-(4-methylphenyl)sulfanylethynylsulfanyl]benzene Structure](https://image.chemsrc.com/caspic/026/86148-27-8.png)
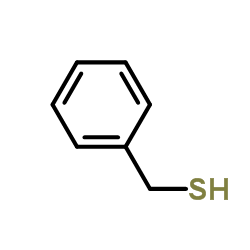


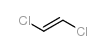
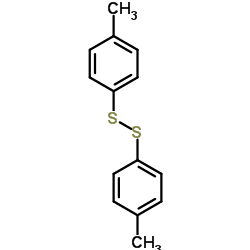

![1-methyl-4-[(E)-2-(4-methylphenyl)sulfanylethenyl]sulfanyl-benzene Structure](https://image.chemsrc.com/caspic/407/5324-85-6.png)
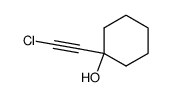
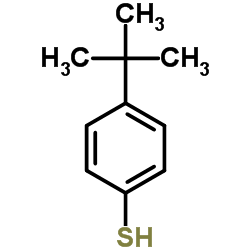
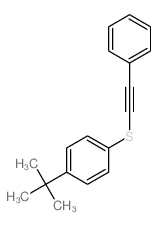
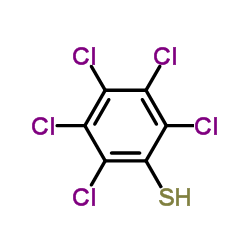
![Benzene,1,2,3,4,5-pentachloro-6-[(2-phenylethynyl)thio] Structure](https://image.chemsrc.com/caspic/260/92030-34-7.png)