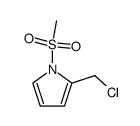
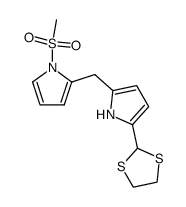
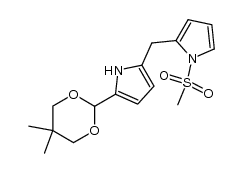
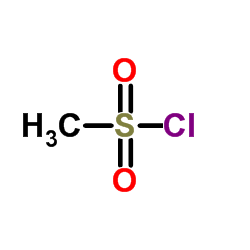
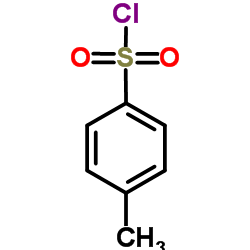
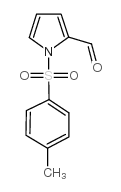
|
~87% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~10% |
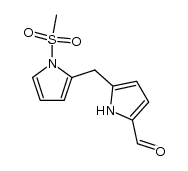
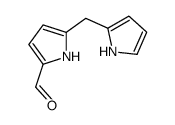

![[1-(Methylsulfonyl)-1H-pyrrol-2-yl]methanol Structure](https://image.chemsrc.com/caspic/140/202580-86-7.png)