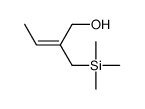
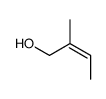
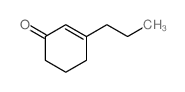
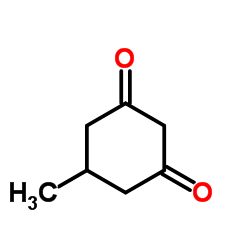
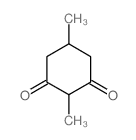
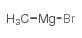|
~7%
Detail
|
|
~94% |
|
~% |
|
~44% |
|
~32% |
|
~69% |
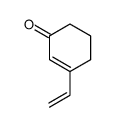
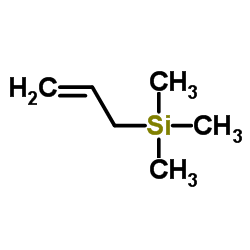
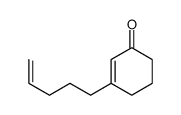
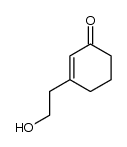
![(Z)-1-[(trimethylsilyl)oxy]-2-[(trimethylsilyl)methyl]-2-butene Structure](https://image.chemsrc.com/caspic/090/100641-10-9.png)