|
~82% |
|
~90% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~93% |
|
~96% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~90% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~88% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~75% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

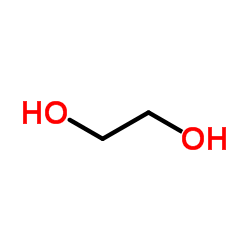
![1,4-DIOXASPIRO[4.4]NON-6-ENE, 6-BROMO Structure](https://image.chemsrc.com/caspic/093/68241-78-1.png)
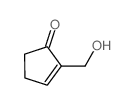

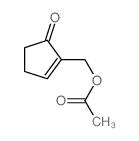
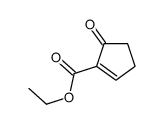
![6-carbethoxy-1,4-dioxaspiro[4.4]non-6-ene Structure](https://image.chemsrc.com/caspic/426/80963-24-2.png)
![2-[[tert-butyl(dimethyl)silyl]oxymethyl]cyclopent-2-en-1-one Structure](https://image.chemsrc.com/caspic/031/68882-72-4.png)
![cis-4,5-dihydroxy-5-[[(tert-butyldimethylsilyl)oxy]methyl]-2-cyclopentenone Structure](https://image.chemsrc.com/caspic/455/68882-74-6.png)
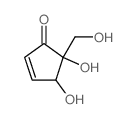
![t-4-[(tert-butyldimethylsilyl)oxy]-5-hydroxy-r-5-[[(tert-butyldimethylsilyl)oxy]methyl]-2-cyclopentenone Structure](https://image.chemsrc.com/caspic/295/80963-32-2.png)
![cis-2,3-dihydroxy-2-[[(tert-butyldimethylsilyl)oxy]methyl]cyclopentanone Structure](https://image.chemsrc.com/caspic/493/68882-73-5.png)
![t-3-[(tert-butyldimethylsilyl)oxy]-2-hydroxy-r-2-[[(tert-butyldimethylsilyl)oxy]methyl]cyclopentanone Structure](https://image.chemsrc.com/caspic/371/80963-30-0.png)

![9-bromo-8-methyl-1,4-dioxaspiro[4.4]non-8-ene Structure](https://image.chemsrc.com/caspic/132/70156-97-7.png)
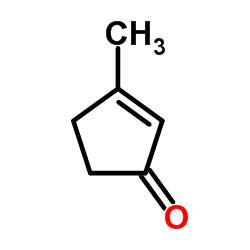
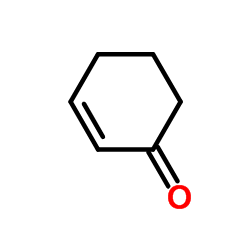
![6-bromo-1,4-dioxaspiro[4.5]dec-6-ene Structure](https://image.chemsrc.com/caspic/094/70156-98-8.png)

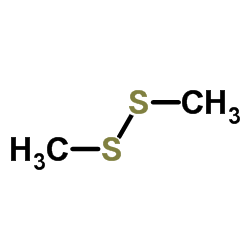
![t-4-acetoxy-5-hydroxy-r-5-[[(tert-butyldimethylsilyl)oxy]methyl]-2-cyclopentenone Structure](https://image.chemsrc.com/caspic/417/68882-75-7.png)
![[5-hydroxy-5-(hydroxymethyl)-4-oxo-1-cyclopent-2-enyl] acetate Structure](https://image.chemsrc.com/caspic/132/68907-80-2.png)
![t-3-acetoxy-2-hydroxy-r-2-[[(tert-butyldimethylsilyl)oxy]methyl]cyclopentanone Structure](https://image.chemsrc.com/caspic/333/80963-31-1.png)
![2-hydroxy-2-[[(tert-butyldimethylsilyl)oxy]methyl]cyclopent-4-ene-1,3-dione Structure](https://image.chemsrc.com/caspic/379/68882-76-8.png)
