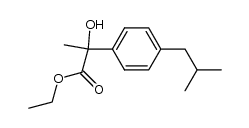
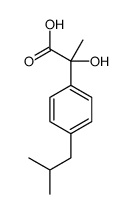
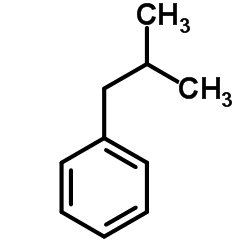
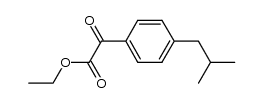
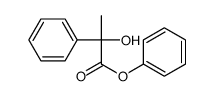
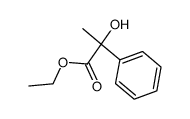
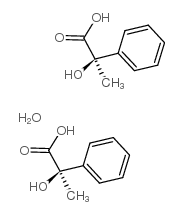
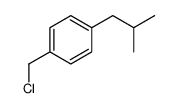
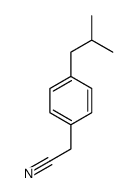
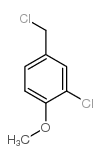
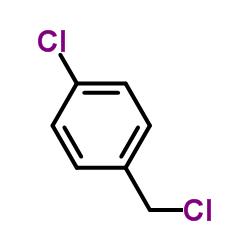
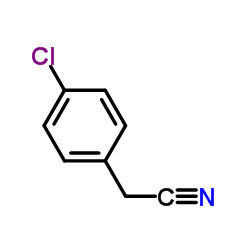
|
~% |
|
~10% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~93% |
|
~90% |
|
~86% |
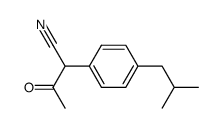
![1-[4-(2-methylpropyl)phenyl]propan-2-one Structure](https://image.chemsrc.com/caspic/425/64758-89-0.png)


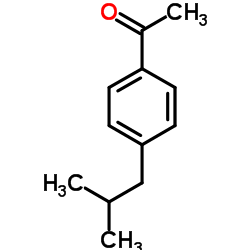
![2-Butenoicacid,3-[4-(2-methylpropyl)phenyl]-(9CI) Structure](https://image.chemsrc.com/caspic/057/91121-60-7.png)