|
~0% |
|
~93% |
|
~% |
|
~% |
|
~% |
|
~% |



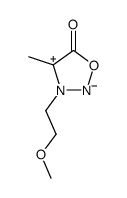
![N-[2-[(4-methylphenyl)thio]ethyl]propanamide Structure](https://image.chemsrc.com/caspic/334/82772-76-7.png)

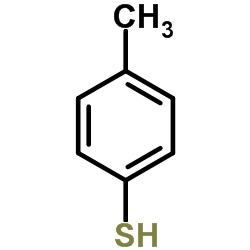
![2-[(4-methoxyphenyl)thio]ethanamine(SALTDATA: FREE) Structure](https://image.chemsrc.com/caspic/110/36155-36-9.png)