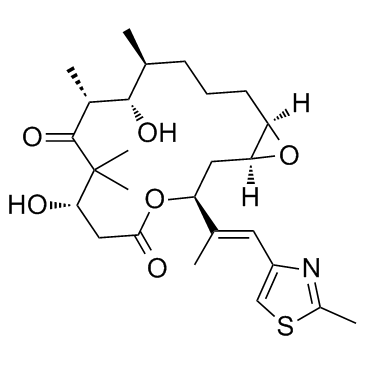
|
~72% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~89% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~66% |
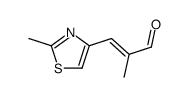

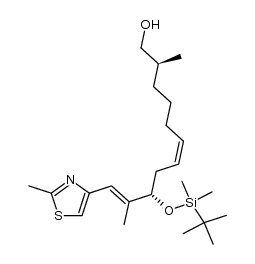
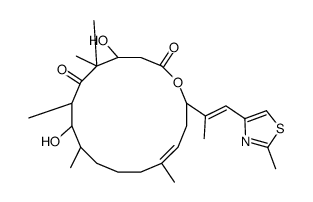
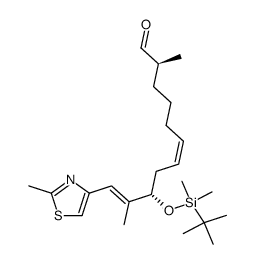


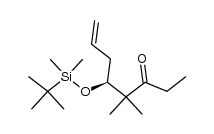
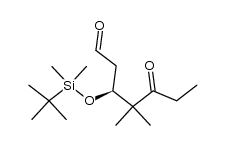
![(5S)-6-[tert-butyl(dimethyl)silyl]oxy-5-methylhexanal Structure](https://image.chemsrc.com/caspic/454/190369-75-6.png)
![(3S,7S,8S,12Z,15S,16E)-3,7,15-tris[[tert-butyl(dimethyl)silyl]oxy]-1-hydroxy-4,4,6,8,12,16-hexamethyl-17-(2-methyl-1,3-thiazol-4-yl)heptadeca-12,16-dien-5-one Structure](https://image.chemsrc.com/caspic/459/193146-53-1.png)
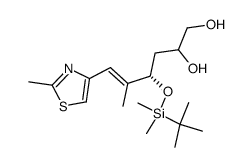
![(5S)-5-{[(tert-butyl)dimethylsilyl]oxy}-7-hydroxy-4,4-dimethylheptan-3-one Structure](https://image.chemsrc.com/caspic/004/250679-53-9.png)
(3Z)-4-methylhepta-3,6-dienyloxy}-1,1,2,2-tetramethyl-1-silapropane Structure](https://image.chemsrc.com/caspic/176/193146-43-9.png)

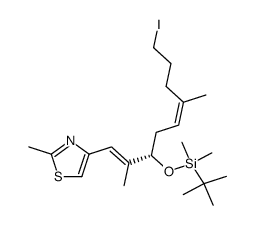
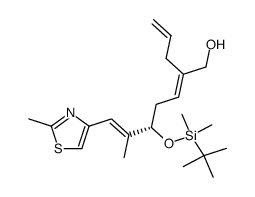
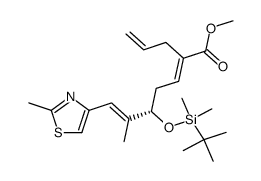
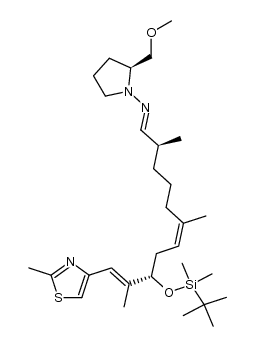
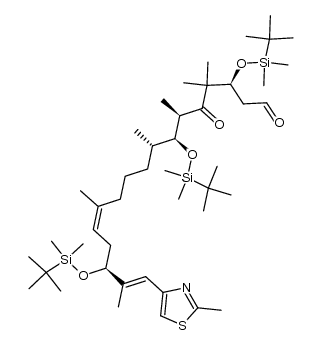
![(3S,7S,8S,12Z,15S,16E)-3,7,15-tris[[tert-butyl(dimethyl)silyl]oxy]-4,4,6,8,12,16-hexamethyl-17-(2-methyl-1,3-thiazol-4-yl)-5-oxoheptadeca-12,16-dienoic acid Structure](https://image.chemsrc.com/caspic/252/193146-63-3.png)
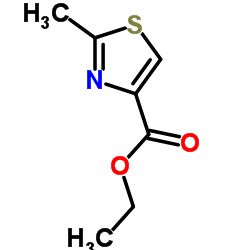
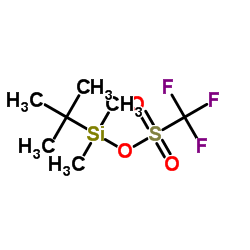
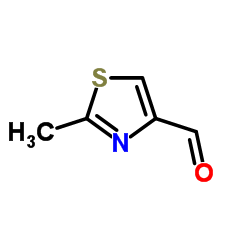
![(3S,7S,8S,12Z,15S,16E)-1,3,15-tris[[tert-butyl(dimethyl)silyl]oxy]-7-hydroxy-4,4,6,8,12,16-hexamethyl-17-(2-methyl-1,3-thiazol-4-yl)heptadeca-12,16-dien-5-one Structure](https://image.chemsrc.com/caspic/438/193146-49-5.png)

![(3S,7S,8S,12Z,15S,16E)-1,3,7,15-tetrakis[[tert-butyl(dimethyl)silyl]oxy]-4,4,6,8,12,16-hexamethyl-17-(2-methyl-1,3-thiazol-4-yl)heptadeca-12,16-dien-5-one Structure](https://image.chemsrc.com/caspic/045/193146-51-9.png)
![(5S)-5,7-Bis-{[tert-butyldimethylsilyl)oxy]}-4,4-dimethylheptan-3-one Structure](https://image.chemsrc.com/caspic/298/187527-25-9.png)
![(3S,4E)-3-{[tert-butyl(dimethyl)silyl]oxy}-4-methyl-5-(2-methyl-1,3-thiazol-4-yl)pent-4-en-1-al Structure](https://image.chemsrc.com/caspic/132/188730-08-7.png)