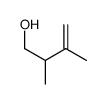
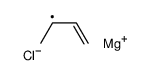
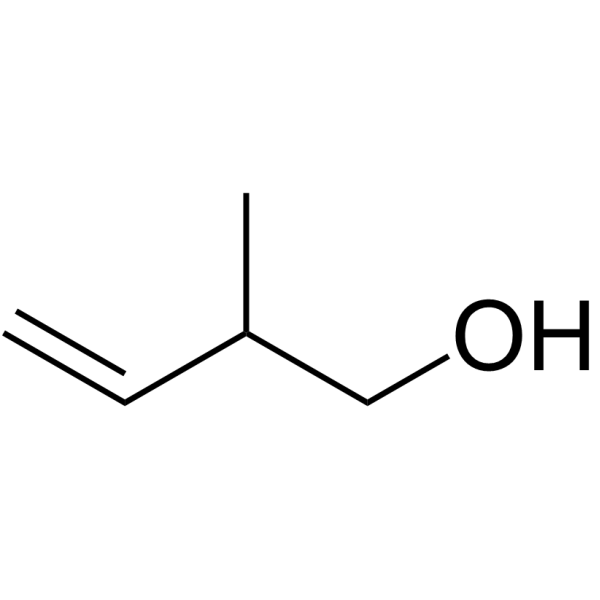
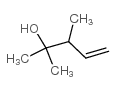
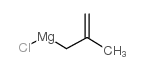
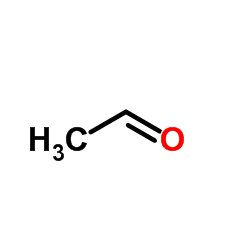
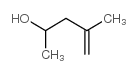
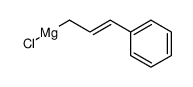
|
~% |
|
~% |
|
~47% |
|
~64% |
|
~71% |
|
~53% |
|
~78% |