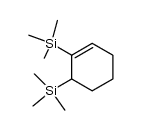
|
~% |
|
~% |
|
~64% |
|
~% |
|
~51% |
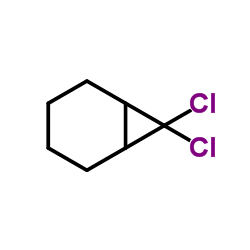
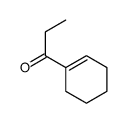

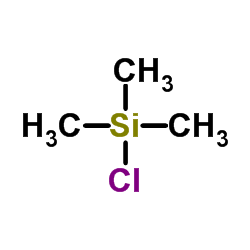
![Bicyclo[6.1.0]nonane,9,9-dichloro Structure](https://image.chemsrc.com/caspic/435/6498-44-8.png)
![trimethyl-(9-trimethylsilyl-9-bicyclo[6.1.0]nonanyl)silane Structure](https://image.chemsrc.com/caspic/166/72907-89-2.png)
![Bicyclo[3.1.0]hexane,6,6-dichloro Structure](https://image.chemsrc.com/caspic/123/23595-96-2.png)
![trimethyl-(6-trimethylsilyl-6-bicyclo[3.1.0]hexanyl)silane Structure](https://image.chemsrc.com/caspic/495/79054-31-2.png)