|
~69% |
|
~85% |
|
~92% |
|
~74% |
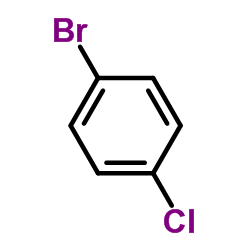
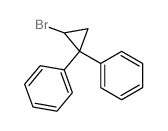
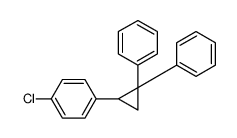



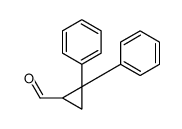
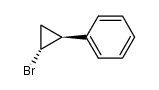

![Methanone,phenyl[(1R,2R)-2-phenylcyclopropyl]-, rel Structure](https://image.chemsrc.com/caspic/210/1145-92-2.png)