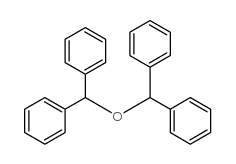
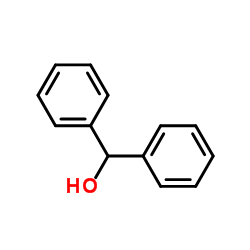
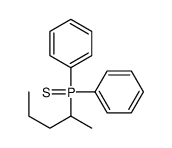
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
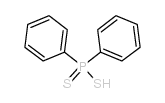

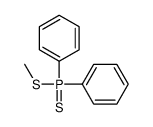
![[methylsulfanyl(phenyl)phosphoryl]benzene Structure](https://image.chemsrc.com/caspic/350/3096-03-5.png)
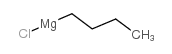
![[butyl(phenyl)phosphoryl]benzene Structure](https://image.chemsrc.com/caspic/100/4233-13-0.png)

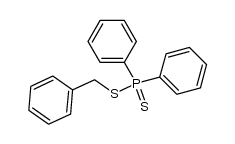

![[diphenylphosphoryloxy(phenyl)methyl]benzene Structure](https://image.chemsrc.com/caspic/352/66004-00-0.png)