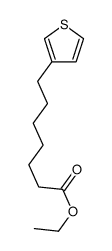
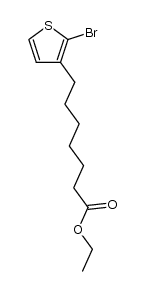
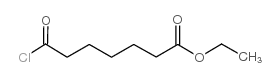
|
~% |
|
~90% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
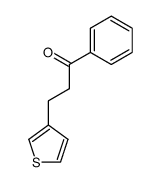
![2,2-dimethyl-7-[3-(3-phenylpropyl)thiophen-2-yl]heptanoic acid Structure](https://image.chemsrc.com/caspic/246/142422-79-5.png)
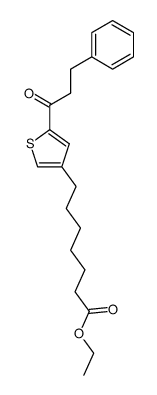
![7-[5-(3-phenylpropyl)thiophen-3-yl]heptanoic acid Structure](https://image.chemsrc.com/caspic/415/142422-48-8.png)