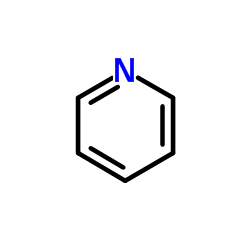
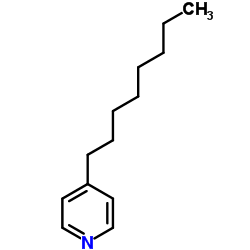
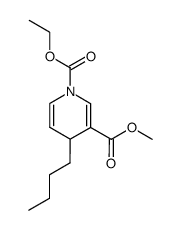
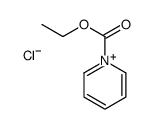
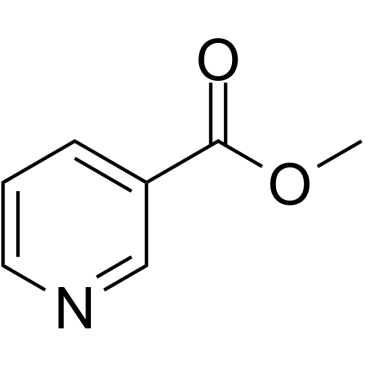
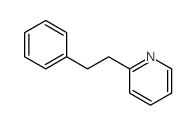
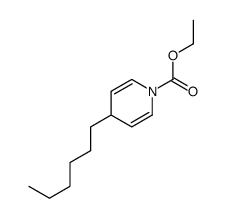
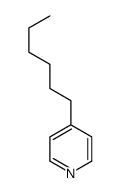
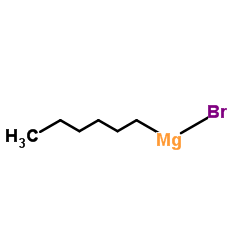
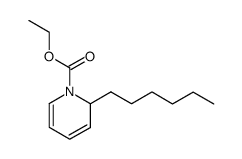
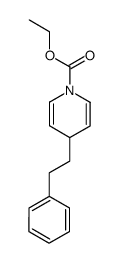
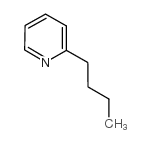
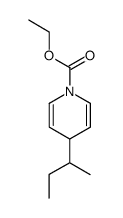
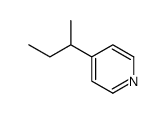
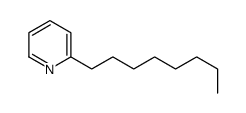
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~66% |
|
~% |
|
~55% |
|
~% |
|
~% |
|
~% |
|
~36% |
|
~% |