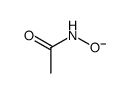
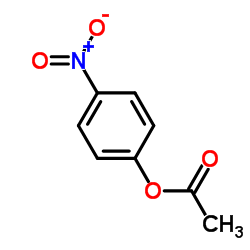
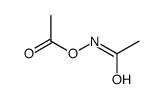
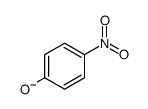
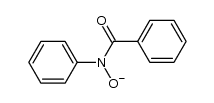
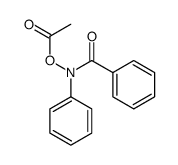
Kinetic and theoretical study on nucleofugality in the pheno...
[Castro, Enrique A.; Canete, Alvaro; Campodonico, Paola R.; Cepeda, Marjorie; Pavez, Paulina; Contreras, Renato; Santos, Jose G. Chemical Physics Letters, 2013 , vol. 572, p. 130 - 135]
|
Kinetic and theoretical study on nucleofugality in the pheno...
[Castro, Enrique A.; Canete, Alvaro; Campodonico, Paola R.; Cepeda, Marjorie; Pavez, Paulina; Contreras, Renato; Santos, Jose G. Chemical Physics Letters, 2013 , vol. 572, p. 130 - 135]
|
Kinetic and theoretical study on nucleofugality in the pheno...
[Castro, Enrique A.; Canete, Alvaro; Campodonico, Paola R.; Cepeda, Marjorie; Pavez, Paulina; Contreras, Renato; Santos, Jose G. Chemical Physics Letters, 2013 , vol. 572, p. 130 - 135]
|
Nucleophilic reactions of molybdate
[Wikjord, Brenda R.; Byers, Larry D. Journal of Organic Chemistry, 1992 , vol. 57, # 25 p. 6814 - 6817]
|
Structure of NPP1, an ectonucleotide pyrophosphatase/phospho...
[Jansen, Silvia; Perrakis, Anastassis; Ulens, Chris; Winkler, Claudia; Andries, Maria; Joosten, Robbie P.; Van Acker, Maarten; Luyten, Frank P.; Moolenaar, Wouter H.; Bollen, Mathieu Structure, 2012 , vol. 20, # 11 p. 1948 - 1959]
|