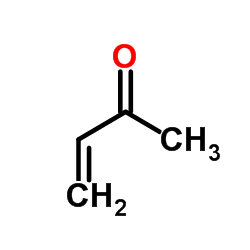
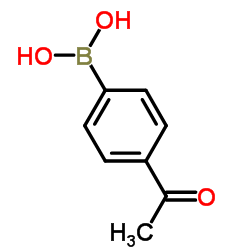
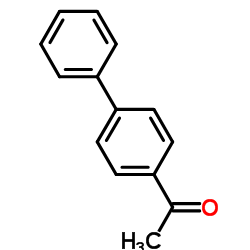
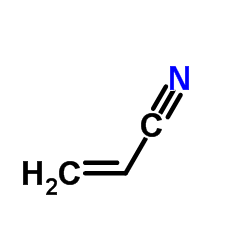
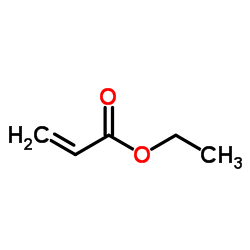
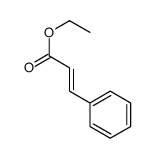
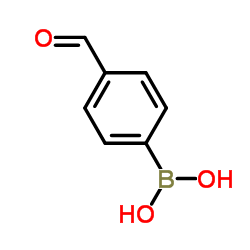
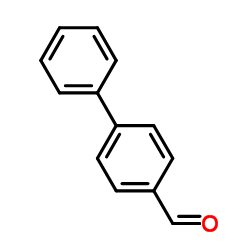
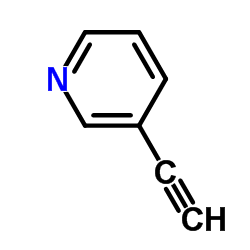
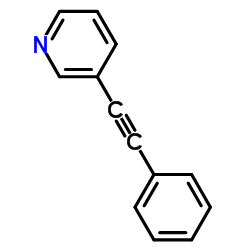
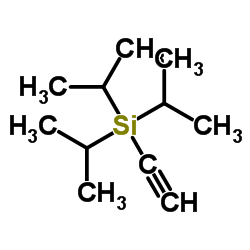
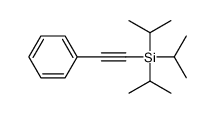
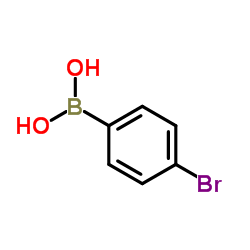
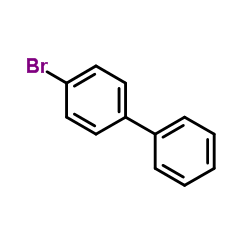
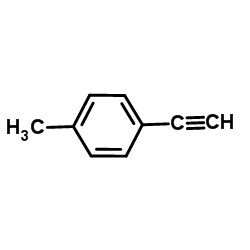
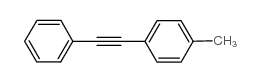
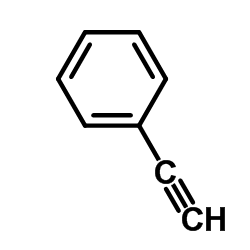
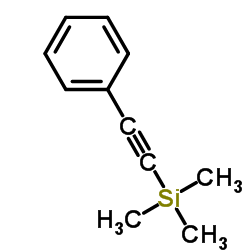
|
~77% |
|
~85% |
|
~95% |
|
~75% |
|
~83% |
|
~76% |
|
~71% |
|
~79% |
|
~70% |
|
~69% |
|
~73% |
|
~78% |
|
~98% |
|
~71% |
|
~60% |
|
~98% |
|
~98% |
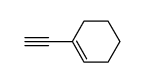

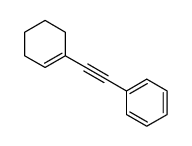
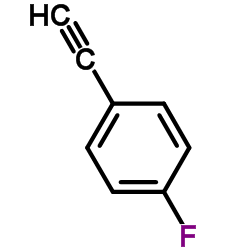
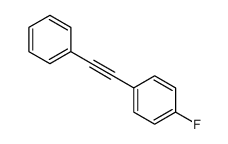
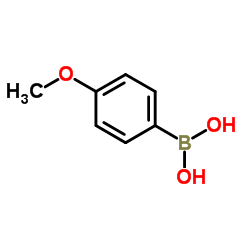
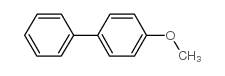
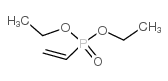
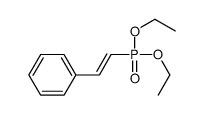
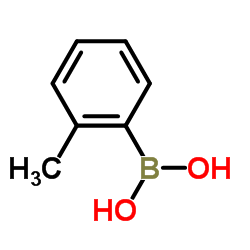

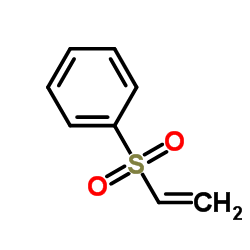
![Benzene,[[(1E)-2-phenylethenyl]sulfonyl] Structure](https://image.chemsrc.com/caspic/405/16212-06-9.png)