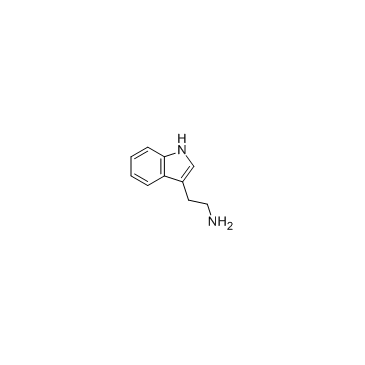
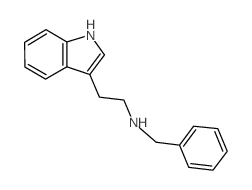
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
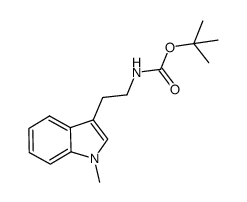
![2,2,2-trifluoro-N-[2-(1-methylindol-3-yl)ethyl]acetamide Structure](https://image.chemsrc.com/caspic/363/111217-41-5.png)
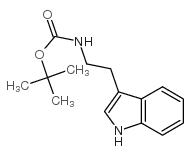
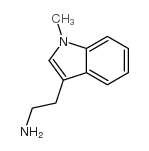
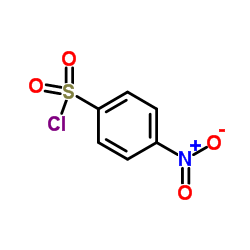
![N-[2-(1-methylindol-3-yl)ethyl]-4-nitrobenzenesulfonamide Structure](https://image.chemsrc.com/caspic/460/919787-23-8.png)