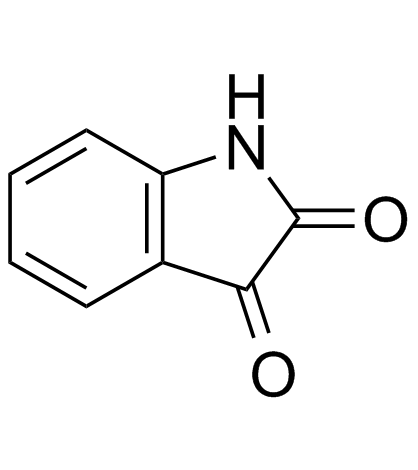
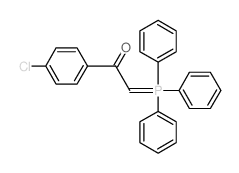
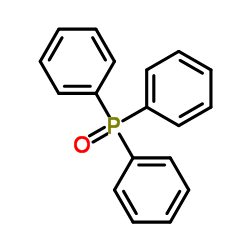
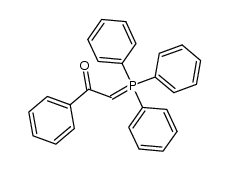
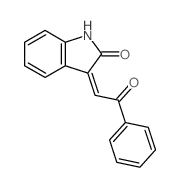
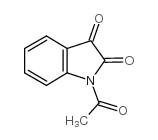
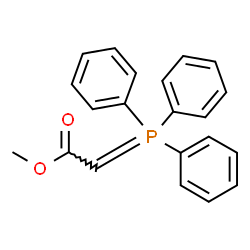
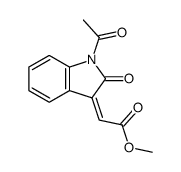
|
~75% |
|
~58% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
![2H-Indol-2-one,3-[2-(4-chlorophenyl)-2-oxoethyl]-1,3-dihydro-3-hydroxy Structure](https://image.chemsrc.com/caspic/494/23241-13-6.png)
![(3Z)-3-[2-(4-chlorophenyl)-2-oxoethylidene]-1H-indol-2-one Structure](https://image.chemsrc.com/caspic/233/23336-93-8.png)