|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

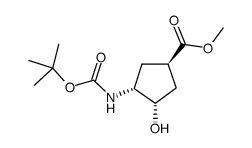

![(1S,2S,4R,5R)-methyl 4-((tert-butoxycarbonyl)amino)-6-oxabicyclo[3.1.0]hexane-2-carboxylate Structure](https://image.chemsrc.com/caspic/145/335668-15-0.png)
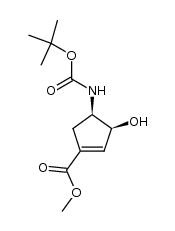
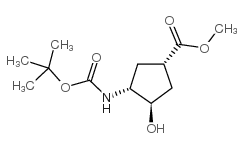
![methyl (1S,3R,4S)-3-{[(tert-butoxy)carbonyl]amino}-4-hydroxycyclopentane-1-carboxylate Structure](https://image.chemsrc.com/caspic/321/321744-14-3.png)
![(1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one Structure](https://image.chemsrc.com/caspic/050/79200-56-9.png)
![Rel-(1S,2R,4S,5R)-tert-butyl 7-oxo-3-oxa-6-azatricyclo[3.2.1.02,4]octane-6-carboxylate Structure](https://image.chemsrc.com/caspic/360/244057-70-3.png)
![3-Oxa-6-azatricyclo[3.2.1.02,4]octan-7-one,(1S,2R,4S,5R)-(9CI) Structure](https://image.chemsrc.com/caspic/063/329910-40-9.png)