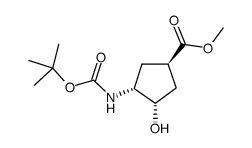
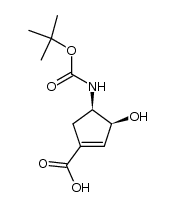
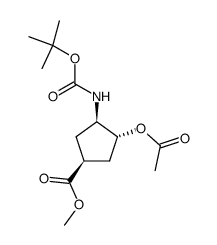
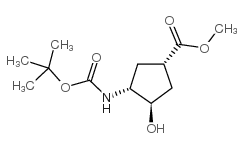
|
~93% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~98% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
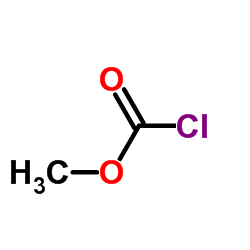
![(1S,3R,4S)-3-({[(1,1-dimethylethyl)oxy]carbonyl}amino)-4-hydroxycyclopentanecarboxylic acid Structure](https://image.chemsrc.com/caspic/454/952708-31-5.png)
![methyl (1S,3R,4S)-3-{[(tert-butoxy)carbonyl]amino}-4-hydroxycyclopentane-1-carboxylate Structure](https://image.chemsrc.com/caspic/321/321744-14-3.png)
![(1R-4S)-4-[[(1,1-dimethylethoxy)carbonyl]amino]- 2-Cyclopentene-1-carboxylic acid methyl ester Structure](https://image.chemsrc.com/caspic/492/251326-99-5.png)
![methyl (1R,3S,4S)-3-{[(tert-butoxy)carbonyl]amino}-4-hydroxycyclopentane-1-carboxylate Structure](https://image.chemsrc.com/caspic/443/262280-14-8.png)
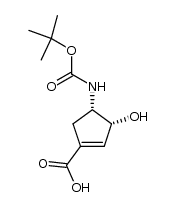
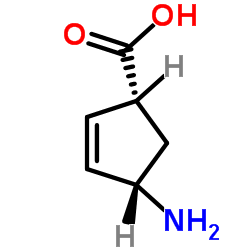
![2-Azabicyclo[2.2.1]hept-5-en-3-one Structure](https://image.chemsrc.com/caspic/088/49805-30-3.png)
![methyl (1R,3S,4R)-3-{[(tert-butoxy)carbonyl]amino}-4-hydroxycyclopentane-1-carboxylate Structure](https://image.chemsrc.com/caspic/152/321744-23-4.png)
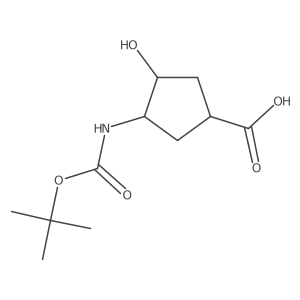
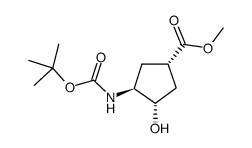
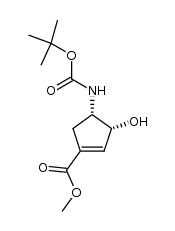
![methyl (1S,3R,4S)-3-hydroxy-4-[(2-methylpropan-2-yl)oxycarbonylamino]cyclopentane-1-carboxylate Structure](https://image.chemsrc.com/caspic/228/321744-21-2.png)
![4-[[(1,1-DIMETHYLETHOXY)CARBONYL]AMINO]-2-CYCLOPENTENE-1-CARBOXYLIC ACID METHYL ESTER Structure](https://image.chemsrc.com/caspic/393/168683-02-1.png)