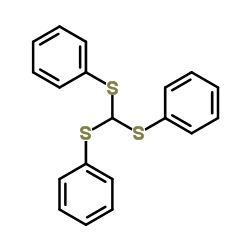
|
~89% |
|
~93% |
|
~88% |
|
~71% |

![Benzene,1-bromo-4-[(2-chloroethyl)thio] Structure](https://image.chemsrc.com/caspic/259/16181-14-9.png)

![Benzene,1,1'-[1,2-ethanediylbis(thio)]bis Structure](https://image.chemsrc.com/caspic/496/622-20-8.png)

![1-chloro-4-[(4-chlorophenyl)sulfanylmethylsulfanyl]benzene Structure](https://image.chemsrc.com/caspic/238/2393-97-7.png)