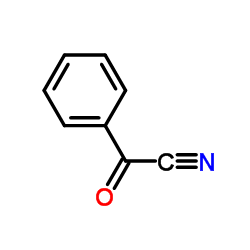
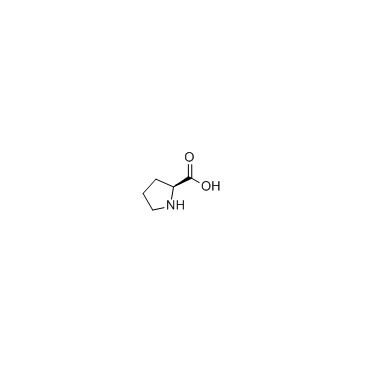
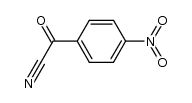
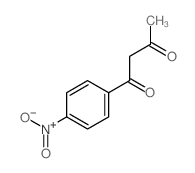
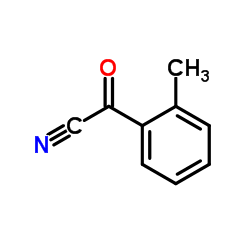
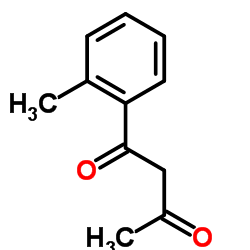
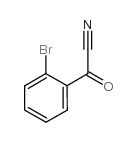
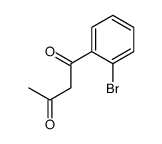
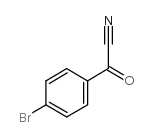
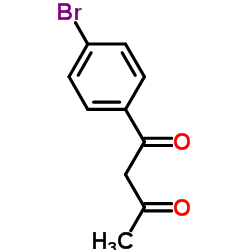
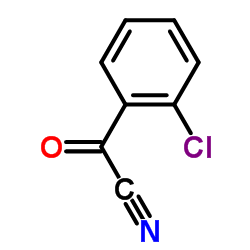
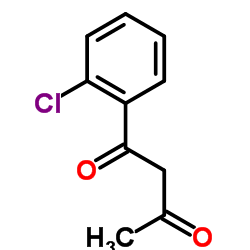
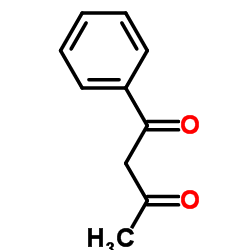
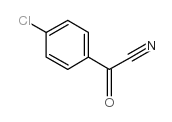
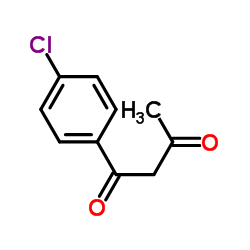
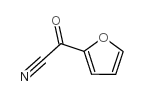
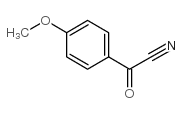
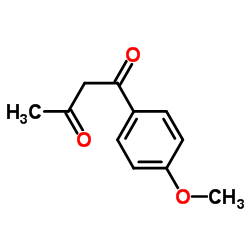
|
~% |
|
~86% |
|
~10% |
|
~88% |
|
~92% |
|
~92% |
|
~% |
|
~91% |
|
~94% |
|
~84% |
|
~68% |