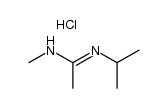
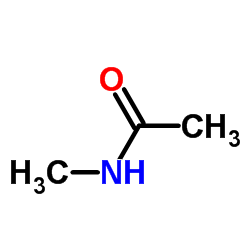
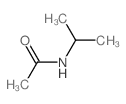
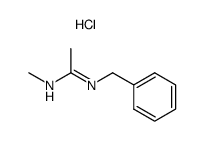
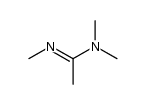
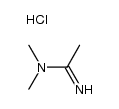
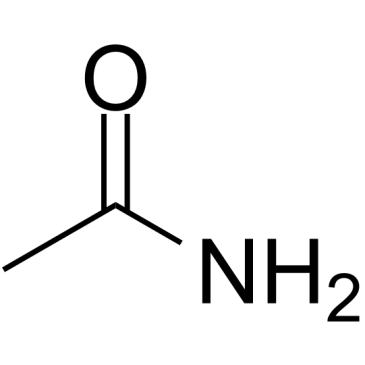
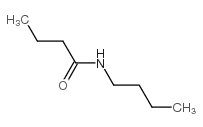
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~2% |
|
~0% |
|
~52% |
|
~42% |
|
~32% |
|
~25% |
|
~22% |
|
~9% |