|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![1-[(1,1'-Biphenyl)-4-yl]-2-methoxy-3,3-dimethyloxiran Structure](https://image.chemsrc.com/caspic/083/408525-79-1.png)
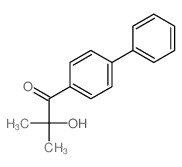
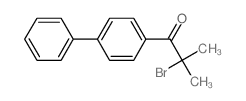
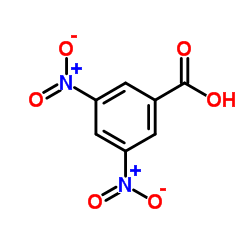
![1,2-Propanediol,1-[1,1'-biphenyl]-4-yl-1-methoxy-2-methyl-, 1-(3,5-dinitrobenzoate) Structure](https://image.chemsrc.com/caspic/179/6976-21-2.png)
![[2-methyl-1-oxo-1-(4-phenylphenyl)propan-2-yl] 3,5-dinitrobenzoate Structure](https://image.chemsrc.com/caspic/152/7472-40-4.png)