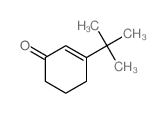
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

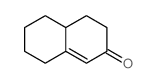

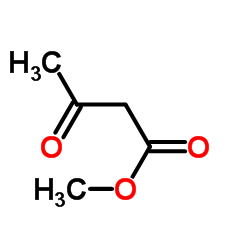

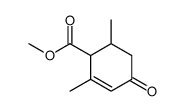

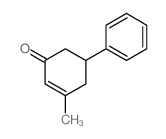
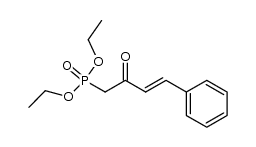
silane Structure](https://image.chemsrc.com/caspic/006/17510-46-2.png)