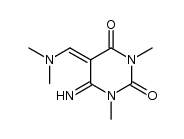
|
~92% |
|
~% |
|
~% |
|
~% |
|
~% |
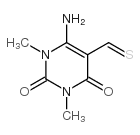
![6-amino-5-[(4-amino-1,3-dimethyl-2,6-dioxopyrimidin-5-yl)methyl]-1,3-dimethylpyrimidine-2,4-dione Structure](https://image.chemsrc.com/caspic/141/10146-98-2.png)