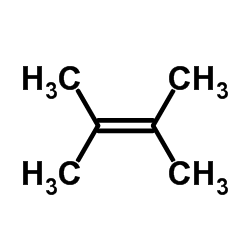
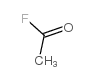
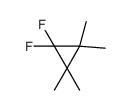
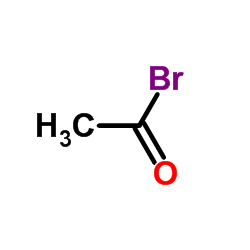
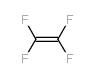
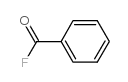
|
~96% |
|
~93% |
|
~86% |
|
~52% |
|
~52% |