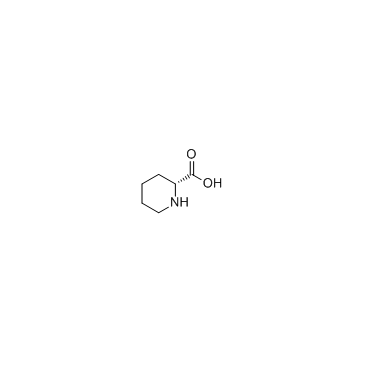
|
~10% |
|
~% |
|
~% |
|
~98% |

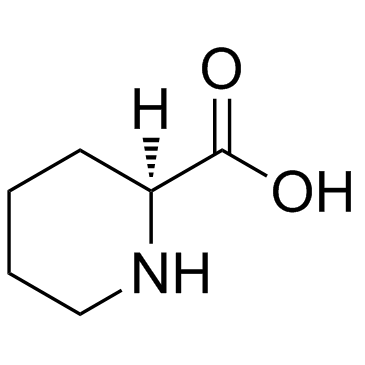
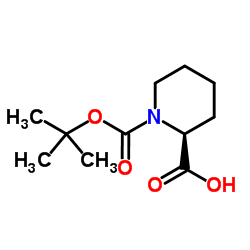
![2-Methyl-2-propanyl 2-[methoxy(methyl)carbamoyl]-1-piperidinecarb oxylate Structure](https://image.chemsrc.com/caspic/257/203056-15-9.png)