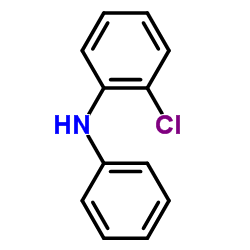
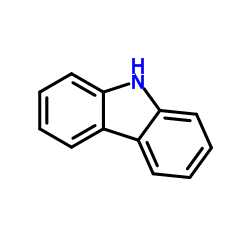
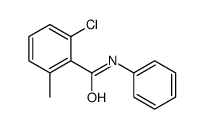
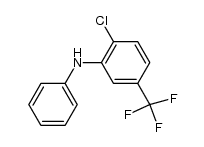
|
~93% |
|
~% |
|
~% |
|
~54% |
|
~90% |
|
~96% |
|
~95% |
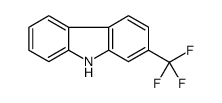
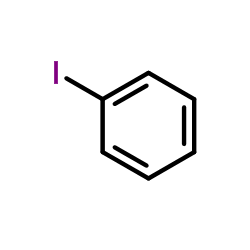


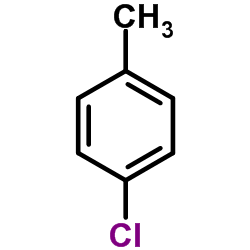
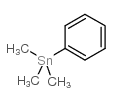
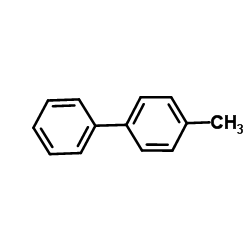
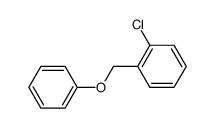
![6H-Dibenzo[b,d]pyran Structure](https://image.chemsrc.com/caspic/147/229-95-8.png)