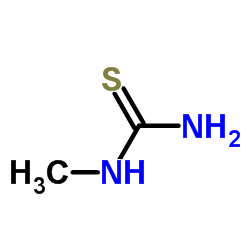
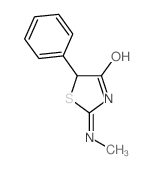
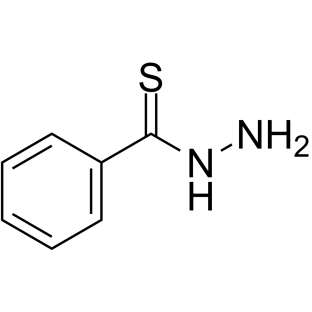
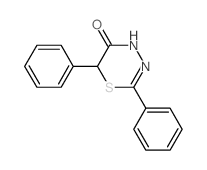
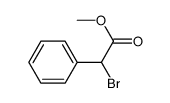
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
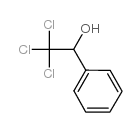
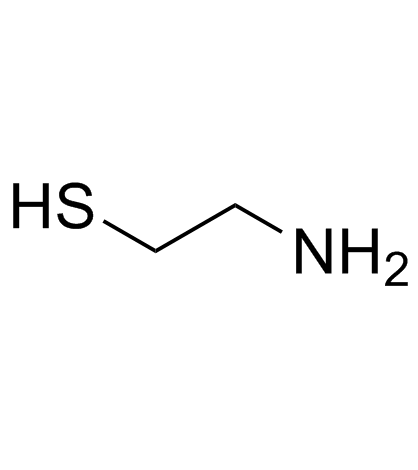
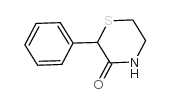
![9-phenyl-10-thia-7-azabicyclo[4.4.0]deca-1,3,5-trien-8-one Structure](https://image.chemsrc.com/caspic/158/38533-19-6.png)