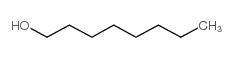
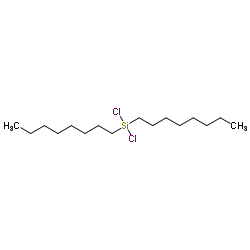
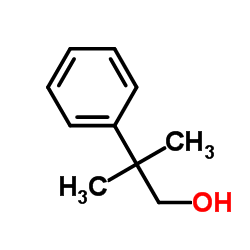
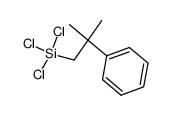
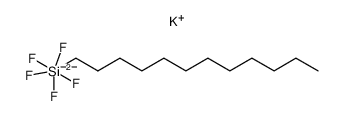
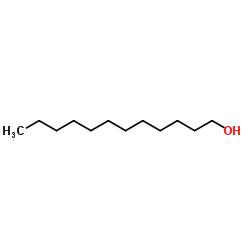
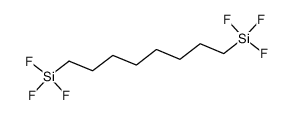
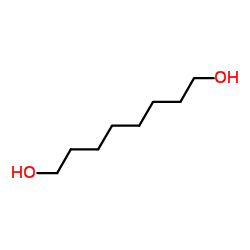
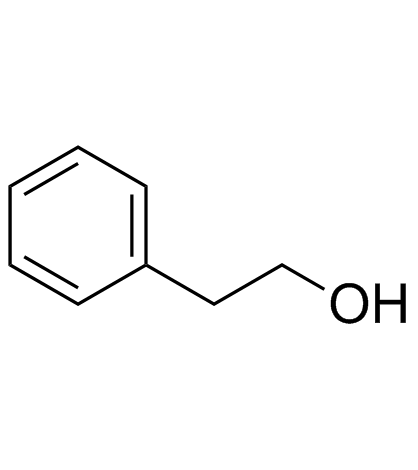
|
~53% |
|
~72% |
|
~% |
|
~74% |
|
~63% |
|
~% |
|
~82% |
|
~74% |
|
~45% |
|
~99% |
|
~67% |
|
~% |
|
~75% |
|
~35% |
|
~80% |
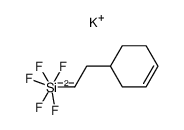
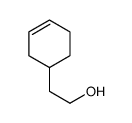
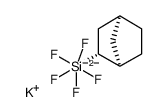
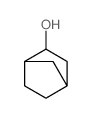
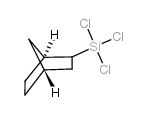
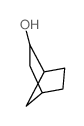
![Silane, bicyclo[2.2.1]hept-2-yltrifluoro-, endo- (9CI) Structure](https://image.chemsrc.com/caspic/481/79745-69-0.png)