|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~40% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~38% |
|
~10% |
|
~55% |
|
~% |
|
~99% |
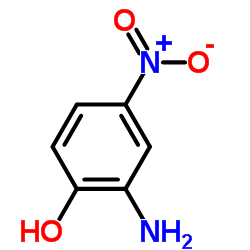
![6-Amino-2,3-dihydro-benzo[1,4]oxazine-4-carboxylic acid tert-butyl ester Structure](https://image.chemsrc.com/caspic/214/928118-00-7.png)
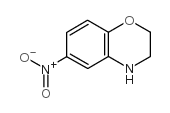
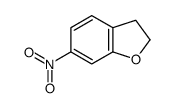
![5-Amino-2,3-dihydrobenzo[b]furan Structure](https://image.chemsrc.com/caspic/399/42933-43-7.png)
![5-acetamido-2,3-dihydrobenzo[1,2-b]furan Structure](https://image.chemsrc.com/caspic/449/81926-25-2.png)
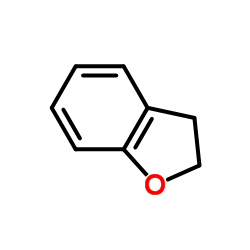
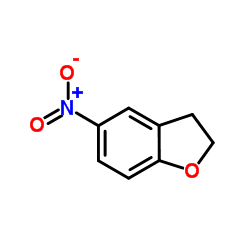
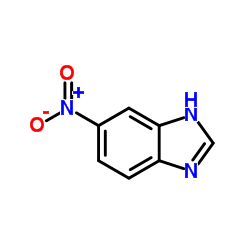

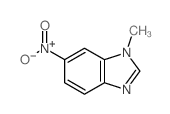

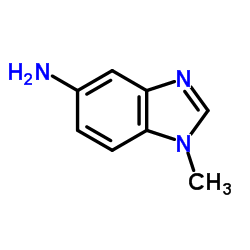
![1-Methyl-1H-benzo[d]imidazol-6-amine Structure](https://image.chemsrc.com/caspic/404/26530-93-8.png)
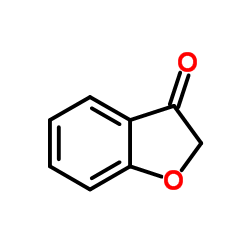
![tert-butyl 5-nitro-1H-benzo[d]imidazole-1-carboxylate Structure](https://www.chemsrc.com/extcaspic/415/1425932-17-7.png)

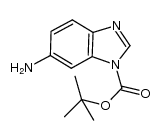
![TERT-BUTYL 5-AMINO-1H-BENZO[D]IMIDAZOLE-1-CARBOXYLATE Structure](https://image.chemsrc.com/caspic/139/297756-31-1.png)
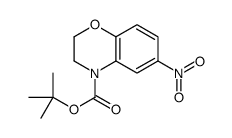
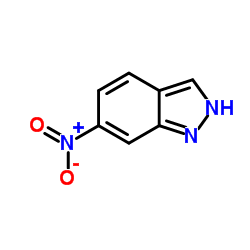
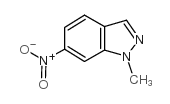
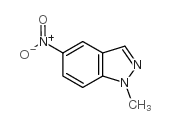

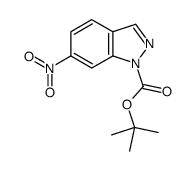
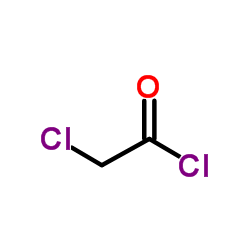
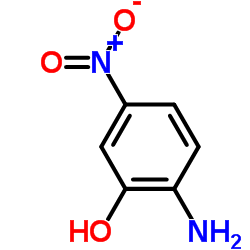
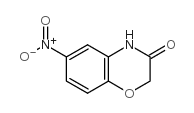
![7-NITRO-2H-BENZO[B][1,4]OXAZIN-3(4H)-ONE Structure](https://image.chemsrc.com/caspic/025/81721-86-0.png)