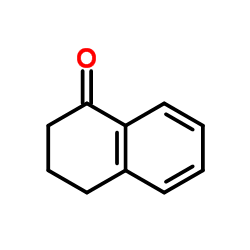
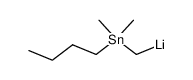
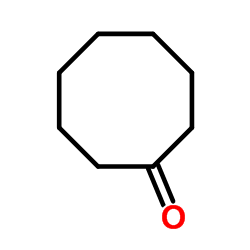
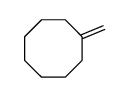
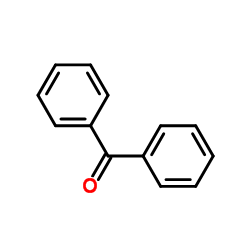
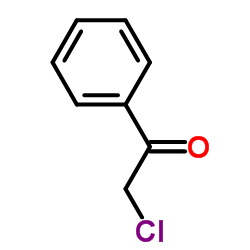
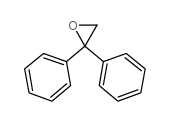
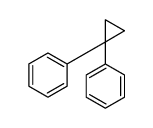
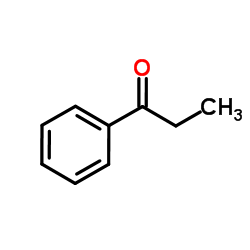
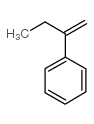
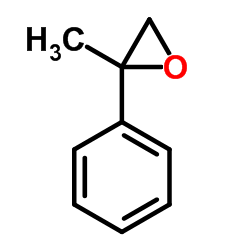
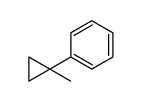
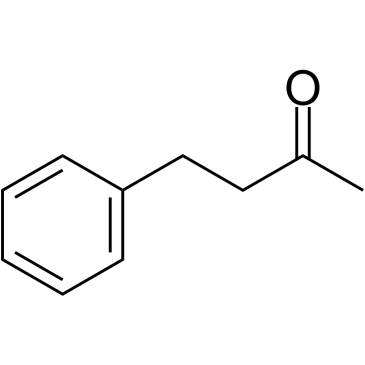
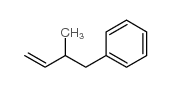
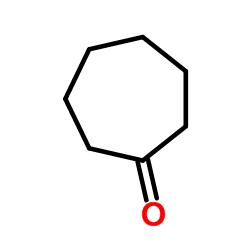
|
~92% |
|
~91% |
|
~62% |
|
~62% |
|
~90% |
|
~86% |
|
~66% |
|
~% |
|
~93% |
|
~94% |
|
~% |
|
~82% |
|
~78% |
|
~78% |
|
~76% |
|
~% |
|
~81% |
|
~81% |
|
~84% |
|
~83% |