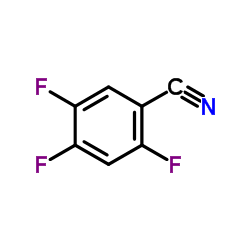
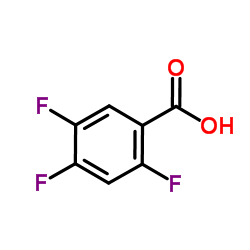
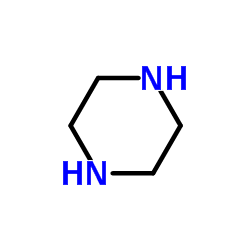
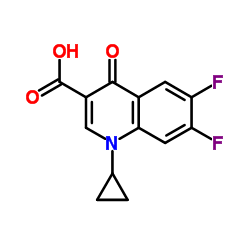
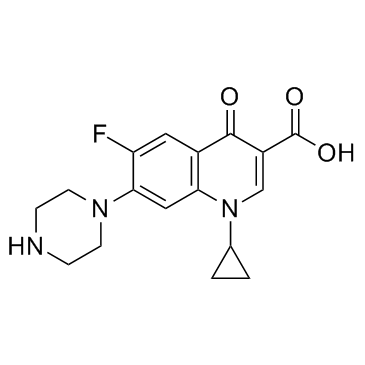
|
~85% |
|
~85% |
|
~73% |
|
~% |
|
~90% |
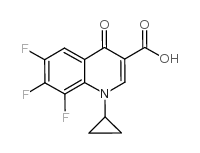
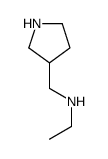
![1-Cyclopropyl-7-{3-[(ethylamino)methyl]-1-pyrrolidinyl}-6,8-diflu oro-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid Structure](https://image.chemsrc.com/caspic/091/99734-97-1.png)
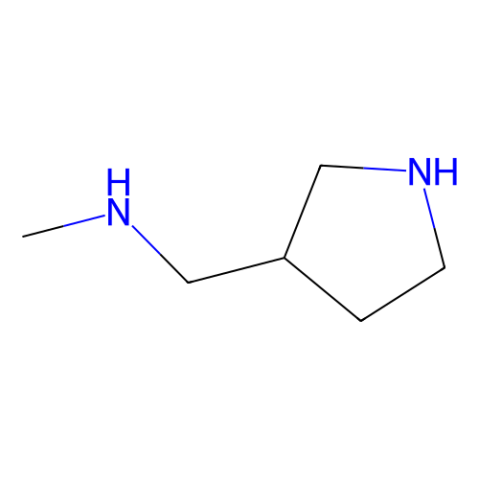
![1-cyclopropyl-6-fluoro-7-[3-(methylaminomethyl)pyrrolidin-1-yl]-4-oxo-1,8-naphthyridine-3-carboxylic acid Structure](https://image.chemsrc.com/caspic/331/113533-65-6.png)
![1-cyclopropyl-6,8-difluoro-7-[3-(methylaminomethyl)pyrrolidin-1-yl]-4-oxoquinoline-3-carboxylic acid Structure](https://image.chemsrc.com/caspic/404/99735-00-9.png)