|
~53% |
|
~82% |
|
~12% |
|
~46% |
|
~% |
![Acetamide,N-[(phenylamino)thioxomethyl] Structure](https://image.chemsrc.com/caspic/042/1132-44-1.png)
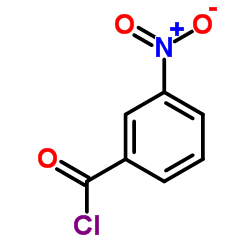
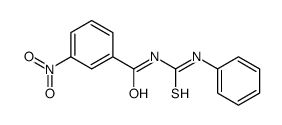
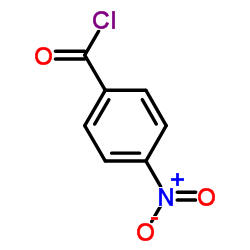
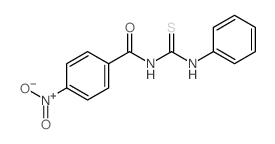
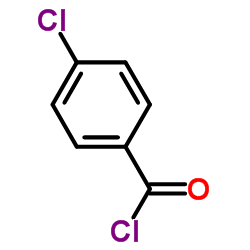

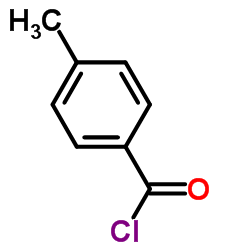
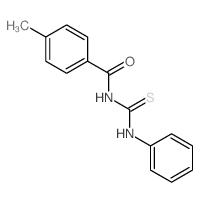
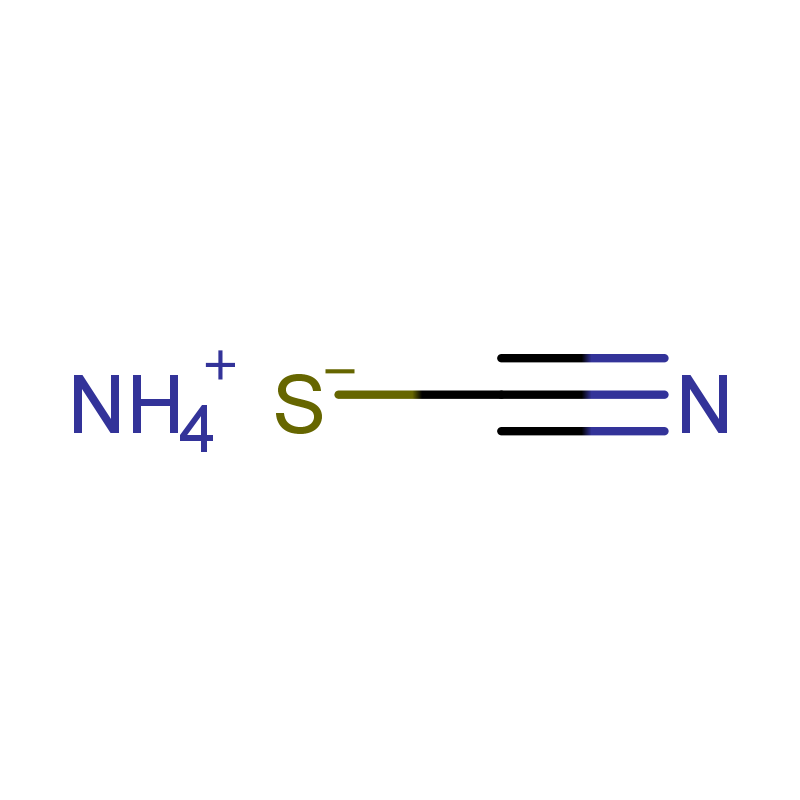

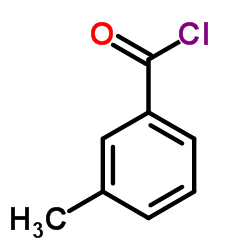
![Benzamide, 3-methyl-N-[ (phenylamino)thioxomethyl] Structure](https://image.chemsrc.com/caspic/370/56437-99-1.png)